Renal and Hepatic Clinical Pharmacology
Our database of patients with renal and hepatic impairment is precisely classified to speed trial enrollment.
160
renal insufficiency studies
140
hepatic impairment studies
CPMI
is a preferred site for sponsors and
CROs to conduct renal and hepatic studies
As outlined in their published guidelines, the FDA mandates that pharmaceutical sponsors conduct pharmacokinetic (PK) studies in patients with impaired renal and hepatic function. With deep experience in both recruiting and executing these trials, ERG is a powerful ally.
Industry-Leading Expertise in Renal and Hepatic Clinical Pharmacology
At ERG, renal and hepatic PK studies are led by one of the country’s leading clinical pharmacology teams trained by Dr. Kenneth Lasseter, a world-renowned expert who is certified by the American Board of Clinical Pharmacology. Our staff are veterans of thousands of clinical trials, with specialized expertise in renal and hepatic trial design and implementation.
ERG’s Experienced Renal and Hepatic Team
Stacy Dilzer
Juan Carlos Rondon, MD
Kenneth Lasseter, MD
Samuel Oberstein, MD
Erica Ridolfi
40% of ERG’s early-phase trials are in patients with renal and hepatic impairment
Targeted Recruitment of Patients with Renal Impairment
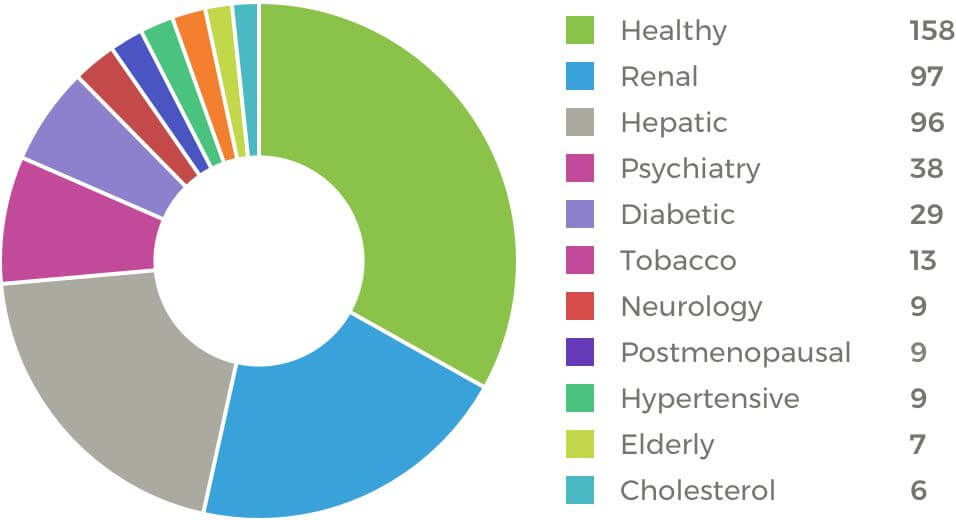
In compiling ERG’s robust list of clinical trial candidates, we conduct extensive screening, enabling us to clearly identify a subpopulation of patients with renal impairment—and to precisely pinpoint the extent of that impairment. Within our primary list, most patients are classified with either mild impairment or moderate impairment. However, since most patients with severe impairment are placed on dialysis, we are able to identify and recruit them through close working relationships with local dialysis centers. Our experience extends to studies with patients with end-stage renal disease (ESRD), requiring collaboration with a dialysis center or other investigator.
Targeted Recruitment of Patients with Hepatic Impairment
Patients with hepatic impairment are divided into two categories: those whose disease is due to alcohol abuse and those whose disease has other causes. ERG has access to patients in every category: with NASH, Child-Pugh A, Child-Pugh B, and even Child-Pugh C. We have strengthened our recruiting capabilities among these populations through active collaboration with key physicians who are pleased to have their patients participate in these trials.
Spotlight
Kenneth Lasseter, MD
- Principal Investigator on 1,800+ clinical studies
- Contributed to 60+ successful NDAs
- Published 300+ articles and abstracts in the area of clinical pharmacology
JZP-110 Study Presented at ACCP
An open-label single-dose study of JZP-110 evaluated the drug’s pharmacokinetic and safety profile in patients with renal impairment; the study found no new safety concerns.
